Group 17 Periodic Table
Group 17 of the periodic table consists of fluorine F chlorine Cl bromine Br iodine I and astatine At. Facts about tennessine and.

Physical And Chemical Properties Of Group 17 Elements A Plus Topper Chemicalpropertiesgroup17ele Physical And Chemical Properties Chemical Property Physics
The elements that are present in group 17 are fluorine chlorine bromine iodine and astatine.

. The group of halogens is the only peri. Reactivity increases as you move up the group. They are called halogens as they react with metals to produce salts.
Isotopes of an. Hence group 17 are called halogens. Free Shipping on All Orders over 35.
Shop as Usual Save. We Are Committed to Making Your Job Easier - Shop our Endless Aisle. The halogens are found in group 17 of the periodic table the second group from the right hand side just before the inert gases.
The elements in this group are all non-metals. What is Group 17 of the periodic table called. In the modern IUPAC nomenclature this group is known as group 17.
Halogen any of the six nonmetallic elements that constitute Group 17 Group VIIa of the periodic table. The f-block columns between groups 2 and 3 are not numbered. Ad At Your Doorstep Faster Than Ever.
Group 17 Periodic Table Name Alkali metals. A Halogen is a Greek word which means salt-former. Up to 24 cash back Group 17 elements are called Halogens.
Group 17 Periodic Table Element. This chapter summarizes the material students need to know about Group 17 of the periodic table for a standard chemistry course. Get Cash Back on Top of Your Credit Card Rewards.
Group 17 elements form diatomic molecules F2 Cl2 Br2 I2 and At2. Those in 17 are the halogens. Emphatically it exists only for fractions of a second at a time.
The name halogen means salt-producing. The halogens are the elements in Group 17 formerly Group VII or VIIa of the periodic table. Tennessine is extremely unstable.
Each aspect in the group is a metal and has distinct qualities. If playback doesnt begin shortly try restarting your device. All have 7 valence electrons in their outermost energy level.
And those in 18 are the noble gases. Physical and Chemical Properties of Group 17 Elements. These elements all have seven valence electrons.
These elements are grouped together as they have similar properties. Ad Millions of Items on One Easy-to-Use Site with Outstanding Customer Service. These elements are known as halogens.
Elements in the same group of the periodic table have similar chemical properties. Those in 16 are the chalcogens. Halogens are nonmetals with high reactivity.
The elements in Group 17 are. Halogens are the most reactive nonmetals on the periodic table. When halogens react with metals they produce a wide range of salts including calcium fluoride sodium chloride common table salt silver bromide and potassium iodide.
Group 17 of the periodic table is the Halogen Group. Here are the properties of these aspects and ways to determine them in the Regular Dinner table. Elements can be classified as metals metalloids and nonmetals or as a main-group elements transition metals and inner transition metals.
Group of people one of several Periodic Kitchen table has the aspects hydrogen potassium boron and sodium and tin. Fluorine Chlorine Bromine Iodine Astatine. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elementsThere are 18 numbered groups in the periodic table.
For example fluorine reacts explosively with sodium to form sodium fluoride. The halogen elements are fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts. Group 17 elements fluorine chlorine bromine iodine and astatine are collectively known as.
Fluorine is the most reactive of the group. Where is Hydrogen within the Periodic Desk. The term halogen is a Greek word which means salt former because they react with metals to form compounds called salts.
B This is because. They are characterised by having atoms with seven electrons in their outer energy shell. Ad Get Cash Back Rewards Exclusive Coupons from Your Favorite Stores.
Get up to 70 Off Now. Group of people one of many Regular Kitchen table contains the components potassium hydrogen boron and. Many people have most likely wondered this quite query.
They are fluorine F chlorine Cl bromine Br iodine I astatine At and the as yet undiscovered ununseptium Uus. 6 rows In the periodic table the Group 17 elements exist as the second column from the right side. This group is the first one to consist of entirely non-metals.
The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core. Halogens are reactive non-metals. What describes the elements in Group 17.

Halogen Easy Science Periodic Table Halogen Easy Science
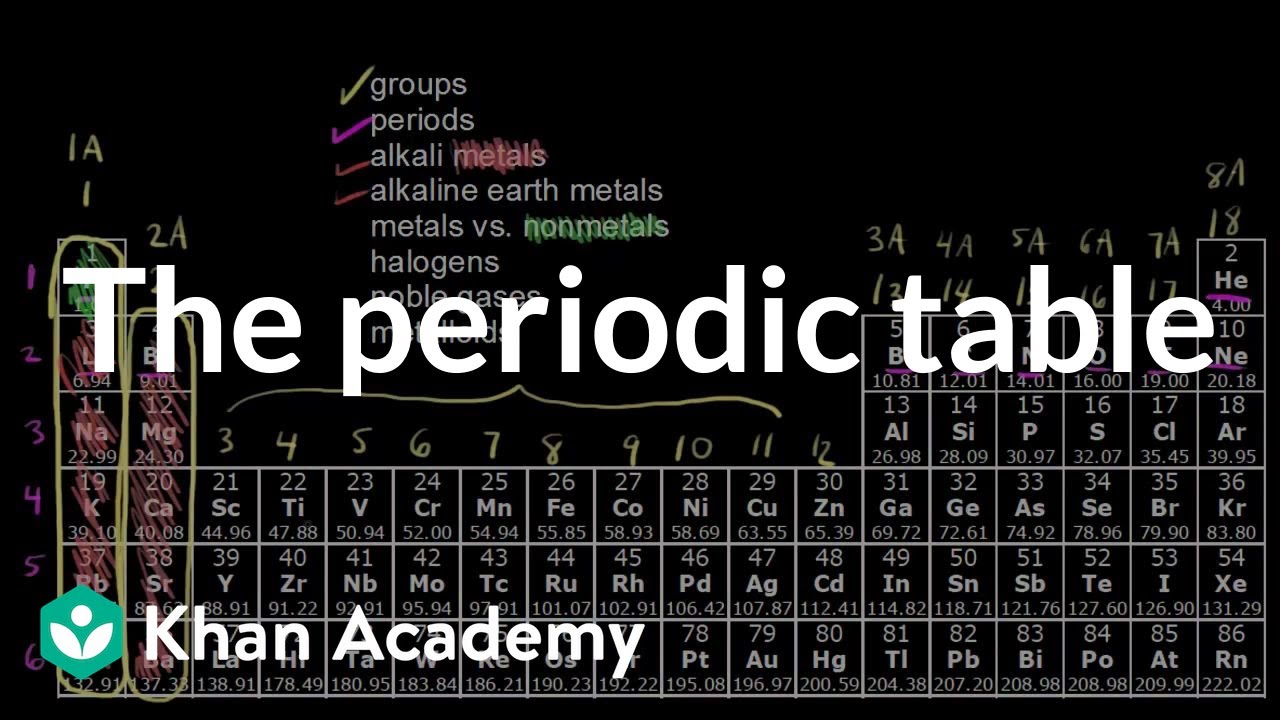
Definitions Of Groups Periods Alkali Metals Alkaline Earth Metals Halogens And Noble Gases How Metals Non Meta Periodic Table Chemistry Chemistry Basics

Periodic Table Groups Perfect Periodic Table With Groups And Families Best Of Periodic Table G Physical Science Middle School Teaching Science Physical Science

Actinoid Element Chemical Element Group Periodic Table Of The Elements Periodic Table Sms Language
0 Response to "Group 17 Periodic Table"
Post a Comment